A good example of the white coats being ghouls with their unsafe and mostly unwarranted methods, and they never counsel you properly on the negative side effects of these scans and X-rays over a lifetime or the cancer risk from them or the contrast agents. The wife’s coworker and friend in the Lord was convinced before dying from cancer that they had given it to her with all the unnecessary breast radiation, and I don’t think X-rays are as safe as they think.
Annual mammograms from ages 40 to 80 are estimated to cause 20 to 25 fatal breast cancers per 100,000 women screened.
And the greatest time of year for malpractice lawsuits is right after new doctors graduate. And I’m aware of one white coat falsely diagnosing patients with cancer so he could profit heavily from treating them…
https://robertyoho.substack.com/p/400-radiology-is-just-as-harmful
Your life should be at risk before you let them touch you.
By Robert Yoho, MD

READER RESOURCES: THE APOCALYPSE ALMANAC: Hidden cures in our dystopian age. Check out the “Cure Cancer in Your Kitchen” chapter. FULLSCRIPT SUPPLEMENTS: top quality and economical.
Table of Contents
- Deadly gadolinium is used as a contrast agent for MRI scans.
- Iodine-based contrast agents, used with CT scans and X-rays, are a lesser evil
- The Radiation Crisis: Cancer on a Population Scale
- CT scans involve significant radiation exposure.
- Fear of lawsuits drives unnecessary imaging.
- Diagnostic Errors Are Epidemic
- MRI Burns, Projectiles, and Other Hazards
- Gadolinium is murder one; the rest of the radiologists’ crimes are shoplifting by comparison.
- Why no one gives a damn
- Appendix: Gadolinium contrast-enhanced MRIs should never be used.
American physicians’ clinical acumen has declined, and the consequences of mistakes have escalated to the point that they need confirmatory scans and blood tests for every decision. Radiologists are happy to cooperate because it makes them money regardless of the risks. The decision of whether to reduce harm to patients or lower the risk of legal trouble is an easy choice for them. So they inject millions of Americans with toxic contrast agents and spray them with unnecessary radiation.
Deadly gadolinium is used as a contrast agent for MRI scans.
This substance is marketed as safe when chelated, but it accumulates in the brains, bones, and kidneys, even when kidney function is normal. The radiology community has known this for over a decade, but continues to use it with minimal disclosure to patients.
Gadolinium is an element on the periodic table and a rare-earth heavy metal. Free gadolinium ions are highly toxic because they compete with calcium for binding sites in biological processes and can cause cellular damage. To make the metal safe for injection, manufacturers bind it to organic molecules called chelators that wrap around the gadolinium ion. The idea was that these chelated forms would pass through the body intact and be excreted by the kidneys within a few hours.
This is untrue. Recent studies reveal that gadolinium deposits accumulate in various organs regardless of kidney function. Post-mortem exams show gadolinium in the brains of patients who had only a few MRI scans. The metal tends to accumulate in the dentate nucleus and globus pallidus, deep brain areas involved in motor skills and thinking. Even the “safest” of these agents, which create the most stable bonds with gadolinium, display this pattern of buildup.
The discovery of how this happens is a breakthrough that radiologists have been slow to acknowledge. Research published in 2024 by the University of New Mexico demonstrates that gadolinium can dissociate from its chelator when it encounters oxalic acid, a compound found throughout the body. Oxalic acid exists naturally in many foods—spinach, rhubarb, nuts, berries, and chocolate—and the body produces it when metabolizing vitamin C supplements.
When gadolinium ions escape their chelators in the presence of oxalic acid, they form gadolinium-oxalate nanoparticles. These tiny crystals penetrate cells and resist removal from the body. These trigger an inflammatory response as the immune system attempts to identify them as foreign metallic invaders. In test-tube experiments, these nanoparticles were formed from both linear and macrocyclic contrast agents, challenging the idea that the more expensive “stable” agents provide sufficient protection.
This mechanism explains why some patients develop severe symptoms after gadolinium exposure while others seem unaffected. People with high oxalate levels from diet or metabolism, or those taking vitamin C supplements, face a higher risk. One gadolinium exposure can trigger devastating symptoms, and nearly half of the patients who developed serious gadolinium-related illness received only a single dose.
The most severe sign of gadolinium toxicity is nephrogenic systemic fibrosis, a condition that can be fatal within months. The skin becomes thick and hard, internal organs develop fibrosis, and joints contract painfully. Some patients have died after just one dose. Because this condition mainly affects individuals with severe kidney disease, the radiology community’s response is to screen for kidney disease and ignore the risk for everyone else.
A new diagnosis has emerged: gadolinium deposition disease. Patients with normal kidney function report chronic pain, cognitive issues, skin changes, and other symptoms that last for years after gadolinium exposure. The radiology community has been slow to recognize this condition, despite the new patient advocacy groups and published case series. Symptoms include bone and joint pain, skin thickening, brain fog, and systemic inflammation, which are patterns seen with chronic heavy metal exposure.
The FDA finally began requiring new warnings on gadolinium contrast agents in 2017, acknowledging that the metal remains in the body but claiming there is no evidence of harm. This is bureaucratic double-speak—the agency admits the toxic metal accumulates but insists patients should not worry. The updated prescribing information now includes a Medication Guide that patients should receive before each injection, though many facilities fail to provide it. The FDA also mandated manufacturers conduct additional safety studies, effectively admitting that decades of use proceeded without adequate safety data.
Radiologists continue to use gadolinium-based contrast agents in 90 million MRI scans each year because the images are sharp and potentially provide valuable diagnostic information. The benefits may outweigh the risks for patients with serious diseases that require accurate diagnosis. However, the situation changes when contrast agents are used excessively or when radiologists do not inform patients about alternatives. Non-contrast MRI techniques are continually improving, and other gadolinium-free contrast agents show promise. Still, gadolinium is the default choice, supported by decades of familiarity and the pharmaceutical industry’s investment.
For a comprehensive indictment of galodinium use, see the Appendix.
Iodine-based contrast agents, used with CT scans and X-rays, are a lesser evil
They avoid gadolinium’s issue of long-term buildup. The body clears iodinated contrast within days through the kidneys. This makes it the preferred option for most imaging that needs contrast enhancement. However, these agents have their own risks, especially for patients with kidney problems.
Contrast-induced nephropathy, now called contrast-associated acute kidney injury, is the primary concern with iodinated contrast. This condition occurs when contrast administration causes a sudden drop in kidney function, usually within 48 to 72 hours. The risk varies greatly depending on pre-existing kidney health. Patients with normal kidney function have a low risk. Those with moderate impairment have an 8 percent incidence, and this rises to 13 percent with moderate to severe impairment, and reaches 27 percent in patients with severe kidney disease.
The radiology community has consistently downplayed these risks. A 2020 consensus statement from the American College of Radiology and the National Kidney Foundation stated that the risk “has been overstated.” This minimization benefits radiologists who face liability when patients refuse contrast-enhanced scans, but it overlooks substantial research showing harm.
Allergic reactions to iodinated contrast occur in about 0.03 percent of cases—about 3 per 10,000 injections. While much less common than reactions to gadolinium, severe reactions need emergency treatment with epinephrine and can be deadly. Delayed reactions, such as skin rashes, occur in up to 14 percent of patients in some studies. The myth that links seafood allergies to iodine contrast reactions persists even though evidence disproves it, showing how little patients understand about the agents being injected into their bodies.
The Radiation Crisis: Cancer on a Population Scale
Conventional X-rays emit relatively low radiation doses and pose minimal cancer risk. A chest X-ray exposes patients to 0.1 millisieverts—equivalent to 10 days of natural background radiation. The cancer risk from this exposure is essentially immeasurable compared to the 42 percent baseline lifetime cancer risk in the U.S. population. A mammogram emits 0.4 millisieverts, about seven weeks of background radiation. Annual mammograms from ages 40 to 80 are estimated to cause 20 to 25 fatal breast cancers per 100,000 women screened.
The radiology community claims mammography screening reduces breast cancer mortality by 24 percent, but this is bulls**t. For screening mammograms in asymptomatic patients, mortality increases with no corresponding statistically significant patient benefit. The following is excerpted from the “Screening Tests are Useless” chapter of Butchered by “Healthcare.”
DO MAMMOGRAMS SAVE LIVES?
This is a politically correct feminist issue that consumes untold time and money. An extensive Cochrane review concluded that this testing does not improve breast cancer survival. This view is supported by others1.
Recently, independent panels in both France and Switzerland recommended abandoning screening mammography. The Swiss are eliminating their program. No study has ever shown a decrease in overall (absolute) deaths with mammography.
A JAMA study showed an increased discovery of breast cancers using mammograms, but no change in total deaths, which is the absolute risk. They said, “These findings suggest widespread overdiagnosis.” For all the frenzied publicity about mammograms, breast biopsies, occasional aggressive surgery, and other treatments, they produce no gain for women.
The following graph2 shows this. Between 1992 and 2015, there was no consequential decline in breast cancer deaths (lower line). All the money thrown at screening programs has just produced a slight rise in diagnoses (upper line):
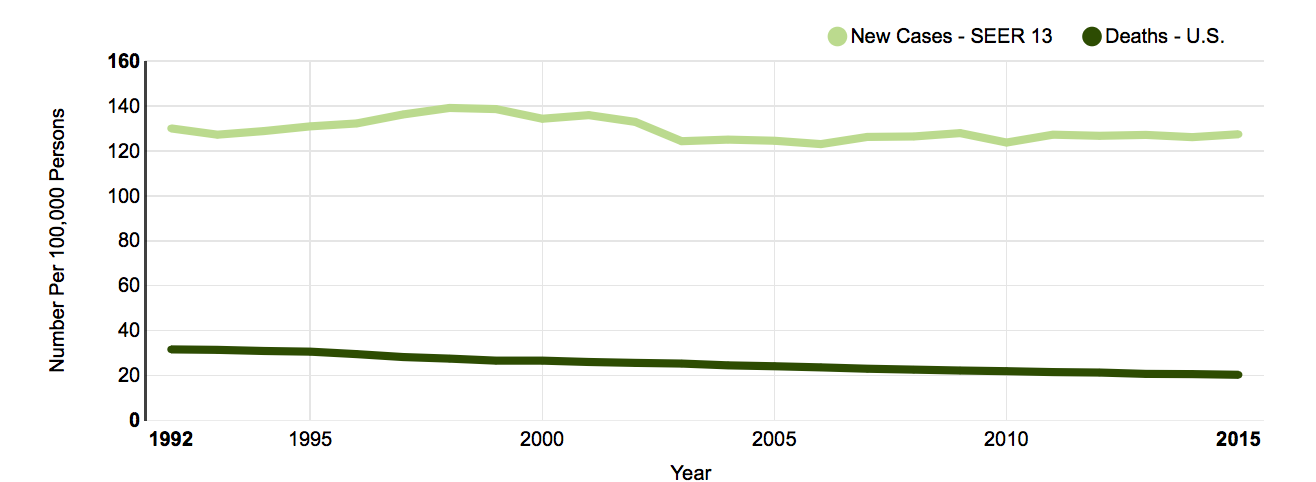
Even when given the same films repeatedly, radiologists read mammograms inconsistently. The process does not reveal cancers accurately, nor does it conclusively eliminate the possibility of cancer.
The standard recommendation is to biopsy any suspicious area. This promotes a cycle of diagnostic and therapeutic misadventure. Pathologists look at the specimens, which are often inconclusive. The result is worry, follow-up, and more surgery.
The process is futile with “ductal carcinoma in situ” (DCIS or intra-ductal carcinoma). A mammogram detects it, then a biopsy makes the diagnosis. The theory is that this is a pre-cancerous or less invasive breast cancer, so it is treated aggressively. Lump removal with radiation therapy is routine, and sometimes surgeons perform a double mastectomy.
A DCIS study of 120,000 patients undergoing various surgeries and radiation treatments showed few differences in outcomes between the treatments. The authors said: “The choice of… treatment had a strikingly small impact on breast cancer-specific survival, calling for a more thoughtful and restrained treatment approach for this disease.” Translated: There was only a slight improvement in RELATIVE survival. Since there has never been a study on intra-ductal carcinoma that used a proper (untreated) control group, ABSOLUTE survival improvement is doubtful. It seems likely that not treating the disease at all might have the same ABSOLUTE (all-cause) death rate as treatment.
DCIS appears to be a fake diagnosis (recall, I am not an oncologist). Some studies show that ten years after this condition is found, a patient is slightly more likely to have died from breast cancer but less likely to have died overall. Some suggest calling this finding something other than cancer.
Follow the money. Radiologists profit from mammograms. The surgeons who do the biopsies are wasting resources and taking risks with their patients’ health. The congressional committees that support mammograms are grandstanding to further their careers.
The charities supporting mammography are in on the action. The United Breast Cancer Foundation and the National Cancer Coalition were among the 50 worst charities in America in one analysis3. Over half of the money given to organizations on this list never went for their purported causes. The United Breast Cancer Foundation earned only a 2 out of 4 stars on charitynavigator.org, indicating it underperforms similar charities. The National Cancer Coalition was worse. They are on the advisory list, indicating significant concerns about illegal activity, improper conduct, or organizational mismanagement.
Gøtzsche has done an exhaustive analysis of the breast cancer numbers for both a Cochrane review (2013) and his Mammography Screening book (2012). He says that screening mammograms and even physician breast exams do not increase survival, and he is outspoken in his opposition to the current approach. He writes that women who discover their own breast lumps are the only ones who should have medical attention.
1 David Newman (Hippocrates’ Shadow, 2008) and a comprehensive 2013 NY Times review by Peggy Orenstein both agree.
2 US National Cancer Institute
3 Tampa Bay Times and The Center for Investigative Reporting
CT scans create significant radiation exposure.
They deliver 50 to 200 times the radiation of standard X-rays. A chest CT exposes patients to 7 millisieverts—70 times the dose of a chest X-ray and equivalent to two years of natural background radiation. An abdominal CT delivers 10 millisieverts, roughly equivalent to about 3 years of background exposure. For any individual patient, a single CT scan raises lifetime cancer risk by approximately 0.05 percent, or one extra cancer per 2,000 scans. This risk seems minimal when compared to the diagnostic benefits.
The picture shifts significantly when millions of scans are added up. A 2025 study published in JAMA Internal Medicine estimated that 93 million CT scans performed in 2023 will eventually lead to 103,000 cancer diagnoses—about 5 percent of all cancers diagnosed in a single year. This places CT scan-related cancers alongside alcohol consumption and obesity as major population-level cancer risk factors, with cigarette smoking surpassing them at 19 percent of all cancers. The rise of CT scanning has made medical imaging the second-largest source of radiation exposure to the U.S. population after natural background radiation, accounting for 24 percent of all radiation exposure.
The most common cancers linked to CT scans include lung cancer, followed by colon cancer. Abdominal and pelvic CT scans account for nearly 40 percent of projected cancers, despite making up a smaller portion of all scans. Children face significantly higher risks because their rapidly dividing cells make them more susceptible to radiation damage, and they have more years ahead for cancers to develop. Studies show that two to three head CT scans can nearly triple the risk of brain tumors in children, while five to ten head scans can double the risk of leukemia. Yet, one-third of children who undergo CT scans have had at least three scans.
The risk increases with multiple exposures. Adults who undergo five or more CT scans face a 2.7 percent higher lifetime cancer risk above the 42 percent baseline. Those who have 22 or more scans see their risk rise to 54 percent—a 12 percent absolute increase. Radiation doses during CT scans vary widely, up to 10 times for the same exam type, depending on scanner settings and operator skill. Many facilities use higher radiation doses than necessary due to misconfigured equipment or inadequate training. This variability means thousands of patients receive excessive radiation that could be avoided with better protocols.
Radiologists are aware of these statistics. They know that CT scans cause cancer. The question is whether this knowledge impacts their practice patterns. The evidence indicates it does not. CT scan use increased by 35 percent from 2007 to 2023, exactly when the cancer risks became well-established in medical literature. Much of this growth reflects “low-value, potentially unnecessary imaging,” according to researchers studying the phenomenon.
Fear of lawsuits drives unnecessary imaging.
More than half of physicians admit they order unnecessary tests specifically to avoid potential malpractice claims. In states that implemented malpractice reforms limiting damages, the use of low-value imaging decreased by 21-32%. This link between legal liability and imaging use shows that defensive medicine significantly contributes to the radiation exposure Americans receive.
Seventy-one percent of radiologists have been involved in lawsuits, making radiology the ninth most-sued medical specialty. The leading causes of these lawsuits are diagnostic errors and failure to communicate findings. Eighty percent of radiology malpractice claims are due to misinterpreted clinical tests, and 80 percent of these cases lead to permanent injury or death.
The median award in radiology malpractice cases involving missed cancer diagnoses ranges from $1.75 million to $6.4 million. However, when radiologists harm patients through excessive radiation exposure or contrast agent complications, the causation becomes less clear and harder to prove. A cancer that appears five years after a CT scan cannot be definitively linked to that specific radiation exposure. Gadolinium buildup in the brain causes symptoms that resemble other conditions.
Radiologists protect themselves by ordering more scans, using contrast more freely, and leaning toward over-testing. The quick financial gain from additional procedures and the chance of missing a diagnosis outweigh the long-term harm from radiation and toxic metals. From a purely self-interested view, this approach makes sense. From a public health standpoint, it is a disaster.
Some estimate that unnecessary imaging costs $9 billion a year. Patients cover these costs through higher insurance premiums, out-of-pocket expenses, and the physical toll of needless radiation and contrast exposure.
Studies examining the link between malpractice litigation rates and imaging use confirm this connection. States with high litigation rates have significantly higher use of advanced imaging services among Medicare patients. The link remains even after accounting for other factors that could influence imaging use. When tort reform reduces litigation pressures, imaging rates decrease accordingly. The precise mechanism is that physicians rely on medical imaging as a defensive measure against lawsuits.
Referring physicians exacerbate this issue. For example, emergency medicine doctors, who face their own litigation pressures, order low-value head CTs at significantly higher rates than neurologists. Non-emergency medicine specialists order even more: 8 times as many unnecessary scans. The culture of defensive medicine dominates the medical system, with radiologists enabling this waste.
Diagnostic Errors Are Epidemic
Diagnostic errors occur in about 4 percent of radiologic interpretations. Most of these errors do not harm because they are caught before reaching the patient or because they involve clinically insignificant findings. However, a significant minority leads to delayed diagnoses, inappropriate treatments, and preventable deaths. Communication failures account for up to 80 percent of medical errors, and radiology is particularly prone to communication breakdowns.
The second most common cause of malpractice lawsuits against radiologists is failure to communicate findings effectively. Radiologists find critical issues—such as aneurysms, cancers, fractures—and then don’t make sure the referring doctor acts on this information. Written reports often sit unread in medical records. Phone calls are never made. Patients remain untreated while life-threatening conditions worsen.
One case illustrates the pattern. A radiologist found a 5-centimeter abdominal aortic aneurysm on a CT scan, but only relayed the preliminary finding of “no stones” through a secretary. The written report mentioning the aneurysm never reached the referring physician. Two years later, the patient died when the aneurysm ruptured. The lawsuit was settled for $2.5 million, with $1.25 million divided between the radiologist and the referring physician. This death was entirely preventable with a single phone call.
The structure of modern radiology enables these failures. Radiologists rarely see patients in person. They work in reading rooms, analyzing images on computer screens, and dictating reports that become part of electronic medical records. The personal connection that once motivated doctors to follow up on critical findings has disappeared. Radiologists have become image interpreters rather than physicians, and this transition has cost lives.
Missed cancer diagnoses top the list of successful malpractice claims against radiologists. Breast cancer is the most common, with lung, pancreatic, and ovarian cancers following. These failures often involve subtle signs that an expert can see in hindsight but that a busy radiologist missed on the initial read. The legal issue is whether the radiologist deviated from the standard of care by failing to notice something a reasonable radiologist would have noticed.
This standard is subjective. Radiologists disagree about whether findings are “obvious” or whether missing them amounts to negligence. Studies of observer variability show that two radiologists examining the same mammogram can reach different conclusions about the presence of cancer in up to 20 percent of cases. Yet the legal system expects perfection, or at least something close to it, from these fallible human observers working under time constraints with imperfect images.
MRI Burns, Projectiles, and Other Hazards
MRI machines pose unique dangers unrelated to contrast agents or radiation. Their strong magnetic fields can turn ferromagnetic objects into high-speed projectiles. Items like oxygen tanks, wheelchairs, IV poles, and scissors can become dangerous missiles that harm or kill patients and staff. These incidents happen even with safety rules in place, often because someone bypassed screening or didn’t realize an object had ferromagnetic metal.
Burns are the most common MRI injury, representing 69 percent of adverse events reported to the FDA. Radiofrequency energy used to create images can heat conductive materials, causing severe burns. Sources include medical devices like pulse oximeters and EKG leads, transdermal medication patches with metallic backing, tattoos with metallic pigments, and even modern athletic clothing that contains copper microfibers.
Patients with medical implants face complex risks. Older pacemakers and defibrillators remain absolute contraindications to MRI because the magnetic field can cause malfunction or move the device. Newer MRI-conditional devices can be scanned under specific conditions, but only if the MRI staff knows the exact model and adheres to the manufacturer’s restrictions. Misidentification or protocol violations have led to deaths.
Cochlear implants, neurostimulators, and drug infusion pumps face similar challenges. Each device has specific MRI compatibility ratings that depend on the magnetic field strength. An implant safe at 1.5 Tesla may become dangerous at 3 Tesla. However, busy MRI facilities sometimes fail to thoroughly verify device compatibility, relying on patient questionnaires that patients complete inaccurately because they don’t understand what information is essential.
The heating of implanted wires and electrodes poses an additional risk through the “antenna effect.” Wires of specific lengths—approximately 26 centimeters at 1.5 Tesla or 13 centimeters at 3.0 Tesla—resonate with the radiofrequency field, concentrating energy at their tips. Deep brain stimulator leads, abandoned pacemaker wires, and retained surgical clips can all heat up to dangerous levels, potentially causing brain damage or cardiac injury.
Gadolinium is murder one; the rest of the radiologists’ crimes are shoplifting by comparison.
Gadolinium contrast agents remain widely used despite accumulating in patients’ brains and forming toxic nanoparticles. Changing practice patterns would mean admitting that decades of “safe” use were criminal. The financial interests of manufacturers, who have invested billions in these products, align with those of hospitals, radiologists, and radiology departments.
Although chelation is purported to protect us from it, free gadolinium is poisonous at the same level as mercury. Using gadolinium clinically is precisely analogous to injecting deadly mercury for imaging, shielded only with an intermediate-strength chelator. Even the most bought-off medical authority has been forced to recognize how harmful mercury is, despite efforts to disguise it under different names, such as thimerosal. Yet they let the gadolinium travesty develop under their noses since 1988, when the first chelated combination was FDA-approved for imaging.
Mercury stays in the body for decades, and gadolinium has been detected in bone up to 8 years post-dosing. The jackass inventors persuaded (bribed?) careless radiologists to inject it into millions of people. The entire MRI contrast agent industry is built on its excellent magnetic properties rather than on finding a non-toxic alternative.
What could go wrong?
Why no one gives a damn
True healers have relationships with patients. Radiologists have none, so they are technicians, not physicians. They see you solely as a billing code and a possible liability. Your health is a distant consideration for them.
Hospitals, as always, focus exclusively on net revenue. Complications increase this by generating new charges.
The FDA acts on behalf of industry because over half of its $5 billion operating revenue comes directly from corporate “user fees” incurred during the patent process. (See Butchered by “Healthcare.”)
These groups are all corporate parasites. True healers have relationships with patients.
Selected References
- Smith-Bindman R, Chu PW, Azman Firdaus H, et al. Projected Lifetime Cancer Risks From Current Computed Tomography Imaging. JAMA Internal Medicine. 2025. do:10.1001/jamainternmed.2025.0123
- Henderson B, Wagner BK, Alcantar NA. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magnetic Resonance Imaging. 2025;147:1-8.
- Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. 2016;29:365-376.
- FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. U.S. Food and Drug Administration. December 19, 2017.
- Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499-505.
- Davenport MS, Perazella MA, Yee J, et al. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294:660-668.
- Villalobos A, Nassiri N, Duszak R, et al. Physicians are using medical imaging as a defense against malpractice claims. Journal of the American College of Radiology. 2022;19:1016-1024.
- Berlin L. Radiologic Errors and Malpractice: A Blurry Distinction. American Journal of Roentgenology. 2007;189:517-522.
- Coverys. 80% of radiology-related malpractice claims lead to permanent injury or death. Business analytics report. 2022.
- Gilk TB, Kanal E. Systematic review of MRI safety literature in relation to radiofrequency thermal injury prevention. Journal of Medical Radiation Sciences. 2024;71:147-165.
Radiology is just as sick as the rest of “healthcare.” Be sure you know why you are being subjected to whatever they want to do to you, and say no until your tongue bleeds. And please sign up for paid and visit my affiliate store below.
Appendix: Why gadolinium should never be used.
An academic paper by Robert Yoho, MD
Summary
• Gadolinium contrast agents cause approximately 0.15 to 2.7 deaths per million doses, with serious adverse reactions in roughly 0.03 percent of administrations
• For most diagnostic applications, gadolinium adds marginal incremental value to imaging accuracy, typically improving diagnostic certainty by less than 15 percent over non-contrast MRI
• Gadolinium deposits permanently in brain, bone, and kidney tissue even in patients with normal renal function, with unknown long-term clinical consequences
• Clinical scenarios where gadolinium is claimed essential, such as cardiac scar imaging, increasingly show viable alternatives through advanced MRI techniques and artificial intelligence
• Free gadolinium exhibits toxicity comparable to mercury by disrupting calcium-dependent cellular processes, though chelated forms reduce immediate toxicity substantially
• Mercury was never considered for imaging contrast because gadolinium’s paramagnetic properties with seven unpaired electrons make it uniquely suited for MRI signal enhancement
• The current diagnostic benefit from routine gadolinium use appears insufficient to justify widespread exposure to a persistent, neurotoxic heavy metal
The Scale of Exposure
Since 1988, physicians have administered over 450 million gadolinium-based contrast agent doses worldwide. In current practice, approximately 50 percent of all MRI examinations include gadolinium injection. This is medicine’s largest mass exposure to a neurotoxic heavy metal, and we are only now understanding it is a massive, ongoing mistake.
Immediate Mortality and Morbidity
This varies by agent type. FDA adverse event data from 2004 to 2009 documented 40 deaths unrelated to nephrogenic systemic fibrosis. The incidence per million doses ranged from 0.15 for gadodiamide to 2.7 for gadobenate dimeglumine. One extensive retrospective analysis of 158,439 gadolinium doses found an adverse effect rate of 0.0404 percent, with events deemed severe enough to require epinephrine or emergency department transfer occurring more frequently than with iodinated contrast despite lower overall reaction rates.
Severe adverse reactions occur in approximately 0.03% of administrations. These include anaphylactoid reactions, severe hypotension, and respiratory compromise. While the absolute numbers appear small, they accumulate substantially across millions of doses. The mortality estimate of approximately one death per 100,000 to 370,000 examinations translates to hundreds of preventable deaths annually from a diagnostic procedure with mostly marginal benefit.
Nephrogenic Systemic Fibrosis
Before 2006, gadolinium was considered remarkably safe, but the emergence of nephrogenic systemic fibrosis dramatically changed that perception. This devastating condition causes severe skin thickening, joint contractures, and internal organ fibrosis with high mortality. Over 400 cases emerged in patients with severe renal impairment who received specific gadolinium agents.
The pathogenesis involves prolonged gadolinium retention due to impaired renal clearance, leading to dechelation and release of free gadolinium ions. Tissue deposition triggers fibrosis through mechanisms that remain incompletely understood. Screening patients for renal dysfunction and restricting gadolinium use in this population has largely eliminated new cases since 2009, but the episode revealed that these supposedly inert chelates can cause catastrophic harm.
Brain Deposition and Retention
In 2014, investigators discovered that gadolinium accumulates in brain tissue following repeated contrast-enhanced MRI examinations. The deposition occurs primarily in the dentate nucleus and globus pallidus, appearing as increased signal intensity on unenhanced T1-weighted images. This finding surprised the imaging community because it contradicted the assumption that gadolinium remained chelated and cleared rapidly from tissues.
Animal studies demonstrate that linear gadolinium agents deposit substantially more than macrocyclic agents, with accumulation persisting for at least five months after administration. Postmortem studies of patients who died from nephrogenic systemic fibrosis found gadolinium in all analyzed tissues, with particularly high levels in the kidney, heart, and blood vessels. The metal appears throughout the body, not just in areas of blood-brain barrier breakdown.
The clinical significance remains uncertain. No definitive evidence links brain gadolinium deposition to cognitive decline, neurologic symptoms, or other adverse health effects in patients with normal renal function. However, the absence of evidence is not evidence of absence. Long-term neurotoxicity studies require decades to complete. Meanwhile, millions of patients accumulate brain gadolinium deposits with unknown consequences.
A Mayo Clinic study examined 4,261 cognitively normal adults using detailed neuropsychological testing. No association emerged between prior gadolinium exposure and cognitive decline. This provides some reassurance but cannot exclude subtle effects or harm that manifests only after prolonged follow-up. The metal persists in tissues for years, possibly permanently. Any toxic effects may require decades to become clinically apparent.
Gadolinium produces only a small diagnostic benefit
Gadolinium enhances images. Whether that translates to improved patient outcomes is unlikely. For most indications, contrast adds minimal incremental diagnostic information over carefully performed non-contrast imaging.
In brain tumor imaging, gadolinium helps delineate tumor borders and differentiate tumor types. Yet studies examining dose reduction find that 50-75% of the standard gadolinium dose maintains diagnostic quality in gliomas and meningiomas. This suggests that much of the standard dose is redundant. More strikingly, retrospective studies of pediatric gliomas show that contrast-enhanced sequences offer little additional information beyond non-contrast imaging.
For rectal cancer, multiple studies demonstrate that gadolinium-enhanced sequences do not improve diagnostic accuracy for tumor penetration through the rectal wall or extension into the mesorectal fascia. Radiologists with varying levels of experience showed no improvement in accuracy or interobserver agreement when contrast was added. The study authors concluded that gadolinium adds cost, increases the time required for additional sequences, and may cause permanent harm.
Brain metastases are an area where contrast appears more beneficial. Gadolinium-enhanced MRI is the standard for detecting brain metastases because contrast highlights the breakdown of the blood-brain barrier that characterizes most metastatic lesions. However, emerging techniques such as susceptibility-weighted imaging with gadolinium may detect additional lesions missed by conventional sequences, suggesting that current protocols remain suboptimal despite gadolinium use.
Cardiac Imaging
Cardiac imaging is claimed to be the strongest case for gadolinium use. Late gadolinium enhancement is considered the gold standard for detecting and quantifying myocardial scar tissue. The technique exploits differential gadolinium washout kinetics between normal myocardium and fibrotic tissue. Scars appear bright on T1-weighted images obtained 10 to 20 minutes after contrast injection. Radiologists love looking at these images.
This application is purported to have clinical utility. Scar burden predicts mortality, sudden cardiac death, and arrhythmic events in patients with coronary artery disease. The presence and pattern of late gadolinium enhancement help distinguish ischemic from non-ischemic cardiomyopathy, guide decisions about implantable cardioverter-defibrillator placement, and determine candidacy for cardiac resynchronization therapy. European guidelines recommend late gadolinium enhancement cardiac MRI for ventricular arrhythmia risk stratification.
But even this application’s utility is doubtful. Deep learning algorithms now detect myocardial scar from non-contrast cine images with good to excellent diagnostic performance. Studies using cine cardiac MRI radiomics achieve area under the curve values of 0.74-0.96 for scar detection compared with late gadolinium enhancement. These techniques analyze wall motion abnormalities and tissue characteristics without requiring contrast.
Alternative techniques, such as native T1 mapping and arterial spin labeling, show promise for contrast-free tissue characterization and perfusion assessment. While these methods remain investigational and require validation, they demonstrate that gadolinium-free cardiac imaging works. The question is whether the incremental diagnostic certainty from gadolinium justifies exposing patients to permanent brain deposition of a neurotoxic metal and sometimes death.
Clinical Outcomes and Lives Saved
Data on lives saved by gadolinium-enhanced imaging does not exist. No randomized trials have compared clinical outcomes between patients receiving contrast-enhanced MRI and those receiving non-contrast MRI for most indications. The assumption that better images lead to better outcomes, given the risks, seems absurd to an outsider with no financial interest like me.
In theory, earlier detection of brain metastases might allow treatment before neurologic symptoms develop. For cardiac scar, precise quantification might improve risk stratification and guide device implantation. For certain tumor types, gadolinium enhancement might reveal malignant features that would otherwise be missed.
But how much benefit occurs is speculative. Most contrast-enhanced studies are performed in patients with known disease, in which imaging serves to characterize lesions already detected by other means. The question is not whether gadolinium helps diagnosis but whether it changes management in ways that improve outcomes. The answer to that question is usually no.
Toxicity profile of galodinium versus mercury
Free gadolinium ions exhibit toxicity comparable to mercury through similar mechanisms. Both metals interfere with calcium-dependent biological processes. Gadolinium has an ionic radius nearly identical to calcium, allowing it to substitute for calcium in enzyme binding sites, voltage-gated calcium channels, and other critical cellular machinery. This competitive inhibition disrupts muscle contraction, nerve transmission, and blood coagulation.
Mercury toxicity is well-established. The lethal dose of methylmercury in humans is estimated at 200 milligrams. In rats, the LD50 for methylmercury ranges from 24 to 40 mg/kg, depending on age. Mercury causes severe neurologic symptoms, including paresthesias, incoordination, tremors, and cognitive decline. Chronic exposure leads to irreversible brain damage.
Free gadolinium shares these properties. It inhibits calcium-regulated signaling, affects voltage-gated calcium channels, and induces oxidative stress and apoptosis in exposed cells. The crucial difference is chelation. Gadolinium-based contrast agents bind gadolinium to organic ligands, thereby substantially reducing toxicity by preventing the release of free gadolinium ions. The stability of these chelates determines safety.
Linear chelates like gadodiamide show higher rates of dechelation and gadolinium release than macrocyclic chelates like gadoterate. This explains why linear agents cause more cases of nephrogenic systemic fibrosis and deposit more extensively in brain tissue. But even the most stable macrocyclic agents eventually release some free gadolinium, as evidenced by persistent tissue deposition.
The authors calculated that eliminating all tissue gadolinium would require 78 to 156 years at the enhanced excretion rate. Once you are injected with gadolinium, you are stuck with it.
And although the FDA is suppressing it, Boyd Haley’s potent chelator NBMI is available to treat mercury poisoning. It likely works as well for lead and iron. The best treatment for gadolinium toxicity, HOPO, is still investigational and won’t be available for years, if at all.
Mercury was never considered as a contrast agent.
The reason is that gadolinium possesses unique paramagnetic properties that make it ideal for MRI enhancement. In the 3+ oxidation state, gadolinium has seven unpaired electrons. These unpaired electrons create a magnetic moment that strongly affects the relaxation times of nearby water protons, brightening the signal on T1-weighted images.
Mercury lacks these paramagnetic properties. It cannot generate the same magnetic effects on proton relaxation. While other metals like iron and manganese have been explored as contrast alternatives, none match gadolinium’s combination of high magnetic moment and favorable relaxivity. Gadolinium produces several orders of magnitude stronger enhancement than alternative paramagnetic ions at equivalent concentrations.
The practical impossibility of developing stable mercury chelates for intravenous use further compounded the barriers. Mercury chemistry differs substantially from rare earth lanthanides such as gadolinium. Creating a chelate that could safely deliver mercury to tissues, provide imaging contrast, and maintain stability in vivo would require solving problems that did not arise with gadolinium.
Public awareness of mercury toxicity might have complicated approval, even if technical obstacles had been overcome. The Minamata disaster* and recognition of mercury’s devastating neurologic effects occurred before the development of MRI. Regulators would have faced substantial hurdles approving a mercury-based imaging agent. But these regulatory concerns became moot because mercury could not provide useful imaging contrast regardless of its safety profile.
*A devastating methylmercury poisoning in Japan, caused by the Chisso Corporation dumping industrial waste into Minamata Bay from 1932 to 1968, leading to severe neurological damage, birth defects, and death in thousands of people and animals who ate contaminated fish and shellfish.
Synthesis
Radiologists consider gadolinium-based contrast agents essential for diagnostic imaging, but their widespread use is a travesty. The incremental diagnostic gain over non-contrast imaging is marginal for conditions ranging from rectal cancer to low-grade gliomas.
Cardiac imaging is not a valid exception. Although late gadolinium enhancement improves risk stratification and theoretically guides treatment decisions, emerging techniques using deep learning and advanced MRI sequences achieve comparable diagnostic performance without contrast. The deeper question is whether our treatments are good enough to justify the risks posed by gadolinium. The answer is obviously no.
The long-term consequences remain unknown because the necessary follow-up studies span multiple decades. The metal accumulates in brain nuclei involved in motor coordination and cognitive function. How much harm this deposition causes will not become clear for another generation.
Immediate death from gadolinium reactions, while low in absolute terms, accumulates to hundreds of preventable deaths annually across millions of doses.
Gadolinium enhances images but rarely changes diagnoses in ways that alter patient management and outcomes. For most applications, non-contrast imaging with careful clinical correlation provides adequate information. Possible exceptions, such as detecting brain metastases, are narrow. To believe these scans provide benefit, you must also believe the treatments work well, which is a far-fetched assumption in nearly every current oncologic situation. (See the cancer chapters of Butchered by “Healthcare” if you still believe oncologists.)
The current practice of routine gadolinium administration is a massive uncontrolled experiment in long-term heavy metal neurotoxicity on hundreds of millions of patients without adequate consideration of alternatives or long-term consequences. It is a poison and should be banned.
Selected References
Bellin MF, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. European Journal of Radiology. 2008;66:160-167.
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. The Lancet Neurology. 2017;16:564-570.
Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. American Journal of Roentgenology. 2009;193:1124-1127.
Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228-232.
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285:546-554.
Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. American Journal of Roentgenology. 2011;196:W138-W143.
Semelka RC, Ramalho M, Vakharia A, et al. Gadolinium deposition disease: initial description of a disease that has been around for a while. Magnetic Resonance Imaging. 2016;34:1383-1390.
Wagner B, Khurana P, Sheridan J, et al. Gadolinium-based contrast agents: a comprehensive risk assessment. Frontiers in Toxicology. 2024;6:1339570.
Zhang N, Yang G, Gao Z, et al. Deep learning for diagnosis of chronic myocardial infarction on nonenhanced cardiac cine MRI. Radiology. 2019;291:606-617.
Zou Z, Zhang HL, Roditi GH, Leiner T, Kucharczyk W, Prince MR. Nephrogenic systemic fibrosis: review of 370 biopsy-confirmed cases. JACC: Cardiovascular Imaging. 2011;4:1206-1216.