This is a great example of how the white coats following treatment protocols will kill you and most won’t question it a bit, and the government will give them a financial reward for violating their oath to do no harm. All you who blindly follow what the white coats tell you better start paying closer attention unless you’re in a hurry to meet your maker or have your quality of life severely diminished on your way to a premature death. And the end covers the new one being advertised on TV a lot right now, Pfizer’s Paxlovid, which is another one to avoid (pay attention to the side effects).
Analysis by Dr. Joseph Mercola
Criminal Investigation for Excess Deaths Due to Remdesivir
Fact Checked
- March 10, 2023 Download PDF
Story at-a-glance
- The antiviral drug remdesivir, brand name Veklury, is approved for use against COVID-19 despite research showing it lacks effectiveness and can cause high rates of organ failure
- John Beaudoin is calling for a criminal investigation into remdesivir, citing data that it may have killed 100,000 people in the U.S.
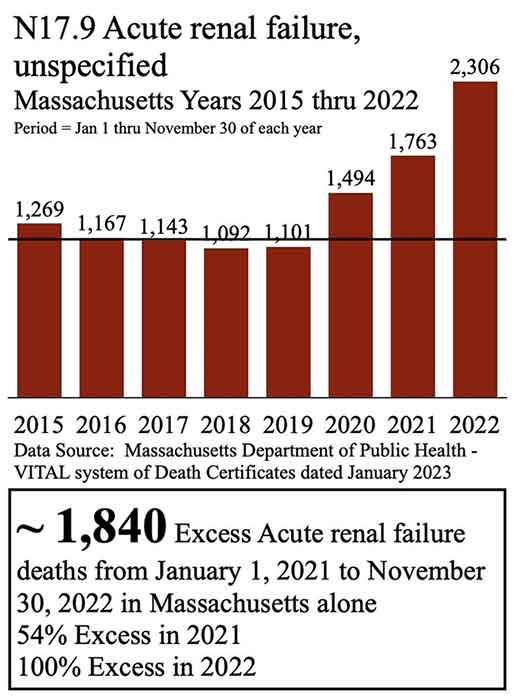
- Beaudoin received all the death certificates in Massachusetts from 2015 to 2022, finding 1,840 excess deaths from acute renal failure from January 1, 2021, to November 30, 2022, which he believes may be due to remdesivir
- A study published in The Lancet found “no clinical benefit” from the use of remdesivir in hospitalized patients
- The U.S. government pays hospitals a 20% upcharge on the entire hospital bill when remdesivir is used
The U.S. Food and Drug Administration authorized the experimental antiviral drug remdesivir, brand name Veklury, for emergency use against COVID-19 in May 2020.1 By October 2020, it had received full approval.2 It remains a primary treatment for COVID-19 in hospitals, despite research showing it lacks effectiveness3 and can cause high rates of organ failure.4
On Twitter, John Beaudoin is calling for a criminal investigation into the drug, citing data that it may have killed 100,000 people in the U.S. “They know,” he says, “or they willfully refuse to know. Either way, it’s homicide.”5
Using drugs that cause organ failure, like remdesivir, isn’t in the best interest of public health. The fact that U.S. health authorities have focused on this and similarly harmful drugs to the exclusion of all others, including older drugs with high rates of effectiveness and superior safety profiles, sends a very disturbing message.
Did Remdesivir Kill Thousands in Massachusetts?
Beaudoin has filed a lawsuit in U.S. District Court and believes a spike in deaths from acute renal failure (ARF) in Massachusetts is due to remdesivir, which is produced by Gilead Sciences. Using a Freedom of Information Act (FOIA) request, Beaudoin received all the death certificates in Massachusetts from 2015 to 2022.
He then graphed the FOIA data, finding 1,840 excess deaths from acute renal failure from January 1, 2021 to November 30, 2022. Beaudoin also revealed an increase in deaths from acute rental failure in every age group over 15 years old, from 2015 to 2022.6 “Thousands dead in Massachusetts ARF likely due to Remdesivir. This requires CRIMINAL investigation,” he tweeted.7
Deaths, Kidney Injury Common With Remdesivir
Remdesivir use didn’t become widespread until 2020. From that time until October 2021, at least 7,491 adverse drug reactions were reported to the World Health Organization’s (WHO) VigiAccess, including 560 deaths, 550 serious cardiac disorders and 475 acute kidney injuries.8
For comparison, only 5,674 adverse drug reactions were reported for ivermectin from 1992 to October 13, 2021.9 Despite its strong safety profile and efficacy, ivermectin was widely vilified during the pandemic. Not to mention, remdesivir costs between $2,340 and $3,120,10 while the average treatment cost for ivermectin is $58.11 Do you think this has anything to do with remdesivir’s promotion and ivermectin’s vilification?
While WHO updated its guidance in April 2022 to recommend the use of remdesivir in “mild or moderate COVID-19 patients who are at high risk of hospitalization,”12 a study published in The Lancet found “no clinical benefit” from the use of remdesivir in hospitalized patients.13 Further, the investigators believed three deaths during the study were related to remdesivir.14
Gilead’s Political Ties Questioned as Remdesivir Use Persists
Still, the question remains why remdesivir continues to be used at all. In November 2020, WHO issued a bulletin recommending against the use of remdesivir in COVID-19 patients, stating, “There is currently no evidence that remdesivir improves survival and other outcomes in these patients.”15
Is it possible that Gilead’s strong political connections have influenced the government’s approvals and recommendations? It’s worth noting that Donald Rumsfeld was the chairman of Gilead from 1997 until he joined the Bush administration in 2001. Rumsfeld had previously served as secretary of defense under President Gerald Ford from 1975 to 1977, and again under President George W. Bush from 2001 to 2006.
FDA Even Approved Remdesivir for Children
In late April 2022, the FDA even approved remdesivir as the first and only COVID-19 treatment for children under 12, including babies as young as 28 days,16 an approval that boggles the mind, considering COVID-19 is rarely serious in children while remdesivir is ineffective and carries a risk of serious, and deadly, side effects.
What’s worse, the drug is also approved for outpatient use in children, which is a first. Dr. Meryl Nass expressed her concerns about the FDA’s approval of remdesivir for outpatient use in babies, stating:17
“The FDA just licensed Remdesivir for children as young as one month old. Both hospitalized children and outpatients may receive it. The drug might work in outpatients, but the vast majority of children have a very low risk of dying from COVID. If 7 deaths per 1,000 result from the drug, as … European investigators thought18 … it is possible it will harm or kill more children than it saves.
Shouldn’t the FDA have waited longer to see what early outpatient treatment did for older ages? Or studied a much larger group of children? Very little has been published on children and remdesivir …
When we look at the press release issued by Gilead,19 we learn the approval was based on an open label, single arm trial in 53 children, 3 of whom died (6% of these children died); 72% had an adverse event, and 21% had a serious adverse event.”
More Lawsuits Filed Against Remdesivir
Two women are suing Kaiser Permanente and Redlands Community Hospital in California for giving remdesivir to their husbands without consent. Both men died from kidney and organ failure after being administered remdesivir. “The day he was admitted on August 12 they started the remdesivir and on [August 17] is when they were done,” Christina Briones told CBS News. “Five doses. [On] the 17th his kidneys started to fail.”20
In California, lawsuits have been filed on behalf of at least 14 families against medical providers for prescribing remdesivir without providing necessary information about it, leading to the patients’ deaths.21 Another wrongful death suit was filed in Nevada, after a patient died of kidney failure and respiratory failure a week after being given remdesivir.22
Safety Signal Revealed for Remdesivir and Kidney Failure
Meanwhile, a study published in Clinical Pharmacology and Therapeutics in April 2021 detected a potential safety signal for remdesivir and acute renal failure:23
“The combination of the terms ‘acute renal failure’ and ‘remdesivir’ yielded a statistically significant disproportionality signal with 138 observed cases instead of the nine expected. ROR [reporting odds ratio] of ARF with remdesivir was 20-fold that of comparative drugs.
Based on ARF cases reported in VigiBase, and despite the caveats inherent to COVID-19 circumstances, we detected a statistically significant pharmacovigilance signal of nephrotoxicity associated with remdesivir, deserving a thorough qualitative assessment of all available data.”
In May 2021, another pharmacovigilance analysis revealed red flags against remdesivir. “Compared with the use of chloroquine, hydroxychloroquine, dexamethasone, sarilumab, or tocilizumab, the use of remdesivir was associated with an increased reporting of kidney disorders,” the study found.24 It concluded:25
“Our findings, based on postmarketing real-life data from >5000 COVID-19 patients, support that kidney disorders, almost exclusively AKI [acute kidney injury], represent a serious, early, and potentially fatal adverse drug reaction of remdesivir. These results are consistent with findings from another group. Physicians should be aware of this potential risk and perform close kidney monitoring when prescribing remdesivir.”
In March 2022, yet another pharmacovigilance analysis warned of a significant association between remdesivir and acute kidney injury, especially in male patients and those over the age of 65 years. “Although causality was not confirmed,” they noted, “the association between remdesivir and AKI should not be ignored, especially in the older, male COVID-19 inpatients.”26
US Government Pays Hospitals to Use Remdesivir
Remdesivir was developed as an antiviral drug and tested during the Ebola breakout in 2014. The drug was found to have a very high death rate and was not pursued further. In the early months of 2020, however, the drug was entered into COVID trials.27 Those trials were also beyond disappointing.28,29,30
Not only was the drug ineffective against the infection but it also had significant and life-threatening side effects, including kidney failure and liver damage.31 Dr. Paul Marik, a pulmonary and critical care specialist and founding member of the Front Line COVID-19 Critical Care Alliance (FLCCC), explained that during the pandemic the only drug he was allowed to prescribe was remdesivir.
When he refused to follow the remdesivir protocol, he was subjected to a “sham review,” an unofficial but well-known process in which a “troublesome” doctor is accused of wrongdoing and basically railroaded out of practice. In the end, he was fired and reported to the National Practitioner Databank and the Board of Medicine.
The financial motivations to report doctors going against the grain run deep. According to Marik, the U.S. government pays hospitals a 20% upcharge on the entire hospital bill when remdesivir is used.32 Citizens Journal also reported that the U.S. government pays hospitals a “bonus” on the entire hospital bill if they use remdesivir.33 It described this practice as a bounty placed on your life, with payouts tied to declining health instead of recovery:34
“For remdesivir, studies show that 71% to 75% of patients suffer an adverse effect, and the drug often had to be stopped after five to 10 days because of these effects, such as kidney and liver damage, and death.
Remdesivir trials during the 2018 West African Ebola outbreak had to be discontinued because death rate exceeded 50%. Yet, in 2020, Anthony Fauci directed that remdesivir was to be the drug hospitals use to treat COVID-19, even when the COVID clinical trials of remdesivir showed similar adverse effects.
… We now see government-dictated medical care at its worst in our history since the federal government mandated these ineffective and dangerous treatments for COVID-19, and then created financial incentives for hospitals and doctors to use only those ‘approved’ (and paid for) approaches. Our formerly trusted medical community of hospitals and hospital-employed medical staff have effectively become ‘bounty hunters’ for your life.”
Officials Push Expensive, Risky Treatments
In addition to remdesivir, Pfizer’s Paxlovid was granted emergency use authorization to treat mild to moderate COVID-19 in December 2021.35 The drug consists of nirmatrelvir tablets — the antiviral component — and ritonavir tablets, which are intended to slow the breakdown of nirmatrelvir.36
But like remdesivir, there are many problems with Paxlovid. In this case, the U.S. Centers for Disease Control and Prevention issued a warning to health care providers and public health departments about the potential for COVID-19 rebound after Paxlovid treatment.37 Further, Pfizer stopped a large trial of Paxlovid in standard-risk patients because it didn’t show significant protection against hospitalization or death in this group.38
Paxlovid costs $529 per five-day treatment39 and has cost U.S. taxpayers $5.29 billion,40 while safe and less expensive options exist. An investigation by Cornell University, posted on the University’s preprint server January 20, 2022, found ivermectin outperformed 10 other drugs against COVID-19.41
Since the FDA and CDC cannot be trusted, and even physicians’ hands are often tied by regulatory red tape, it’s imperative to take responsibility for your own health. In the case of COVID-19, seek early treatment using an effective and safe protocol — not one that puts profits over patients.